The Certainty of being Uncertain
Of all the entire physics the Werner Heisenberg's Uncertainty principle is probably the most mysterious and strange. It kind of tells us that there is a limit to our machines, our efforts and we ourselves can extract about the true nature of the universe. The principle reveals how the universe has concealed its true behavior and nature under the invisible sheet which restricts us from knowing it all together and that why it's my favorite in all of physics.
So enough of chit-chatting there, let's dive right into the principle to unravel the true understanding and working that lies under it.
Well for the definition it goes something like this:
You can't simultaneously measure the position and velocity of a sub-atomic particle with exact precision.
Formula:
After reading the above statement not much would have gone through your brain or maybe on the other hand if you were paying attention even then it might not be that much of a surprise, but the conclusion that this statement delivers is of unbelievable consequences.
So first of all what this statement wants to say is that:
You can only measure either the position or the velocity of a sub-atomic particle at a single time with exact precision. You can't measure both (velocity and position) at the same time and if you try to do so there is a specific amount of uncertainty in the measurement that you have achieved. If you are more certain about the position of the particle then would be that much uncertain about the velocity of the particle.
More particularly, If one knows the exact position of a sub-atomic particle then it is impossible to measure the exact velocity of that same particle at the same time.
So after doing with the explanation of the statement let's get to the interesting part and understand how Heisenberg led to the development of the principle and intuition behind the whole idea.
Explanation:
So let us start with the beginning and first of all, understand how we measure things. So for measuring any quantity whether it is volume, electric charge, mass whatever it is the basic and foremost requirement is the presence of the body whose quantity we have to measure, and also the essential tools, But even if we have all above-mentioned things we obviously need to see the object.
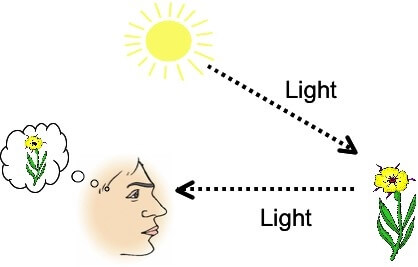
So now how do we see the object and for that matter anything? We see things when light strikes that object and reflects back into our eyes, the reflected light is then processed by the brain that we see in the form of that object.
You would be thinking that why I am telling you all this basic stuff and how it relates to the principle, but bear with me for a while because I am gonna blow your mind.
So for seeing anything the light must strike that object and reflect back into our eyes but with all this, the important part is that the wavelength of the light that strikes the object must be smaller than the object itself.
Light will only reflect back when it strikes an object that is bigger than the light(wavelength of light) itself. Things are all games and fun in real-life objects which are thousands of times bigger than the wavelength of visible light, but sub-atomic particles are very very very small so small that the visible light that we use to see the objects just passes by i.e. the wavelength of the normal visible light is bigger than the sub-atomic particle that we want to see. Now we can solve this problem by using light of a smaller wavelength but a smaller wavelength also means higher energy (since the wavelength is inversely proportional to the frequency and energy of the light)
Now some weird stuff starts to happen and that is the lower wavelength light is a lot more energetic and so energetic that when it strikes that sub-atomic particle it changes its position and when we try to measure it has already changed thereby causing that uncertainty that we talked about. Now, this is an inevitable problem since we want to measure the position of the particle we will have to strike light of a lower wavelength and therefore of higher energy which will eventually change its position and velocity. On the other hand, if we used the light of a higher wavelength we may be able to measure its velocity but not position









If You have any doubts or suggestions, please let me know in the comments.